The U.S. Food and Drug Administration (FDA) leads the Sentinel Initiative. FDA created the Sentinel Initiative to meet a mandate by Congress in the FDA Amendments Act of 2007. Through the Sentinel Initiative, FDA aims to develop new ways to assess the safety of approved medical products including drugs, vaccines, and medical devices.
FDA Sentinel Initiative Infrastructure
The Sentinel Initiative infrastructure supports the Sentinel System and FDA-Catalyst. The CBER Biologics Effectiveness & Safety (BEST) System supports the Sentinel Initiative to assure the safety and effectiveness of biologic products, but operates outside of the Sentinel Infrastructure.
Sentinel System
The Sentinel System helps to answer the FDA's questions on approved medical products. It does this by creating computer programs that analyze electronic healthcare data. These computer programs use statistical methods to study relationships and patterns in medical billing information and electronic health records.
Active Postmarket Risk Identification and Analysis (ARIA) System
Within the Sentinel System is the ARIA System. The ARIA system has two main components. The first component is the healthcare data formatted in the Sentinel Common Data Model. The second component includes computer programs that give FDA the tools to analyze this data. Congress mandated ARIA in the United States FDA Amendments Act (FDAAA) of 2007. ARIA is the most widely used portion of the Sentinel System.
FDA-Catalyst
FDA-Catalyst supplements the Sentinel System. The data FDA-Catalyst provide come from interactions with patients and/or providers. FDA-Catalyst combines this data with data included in the Sentinel infrastructure.
History of the Sentinel Initiative
Below is a timeline of milestones for the Sentinel Initiative.
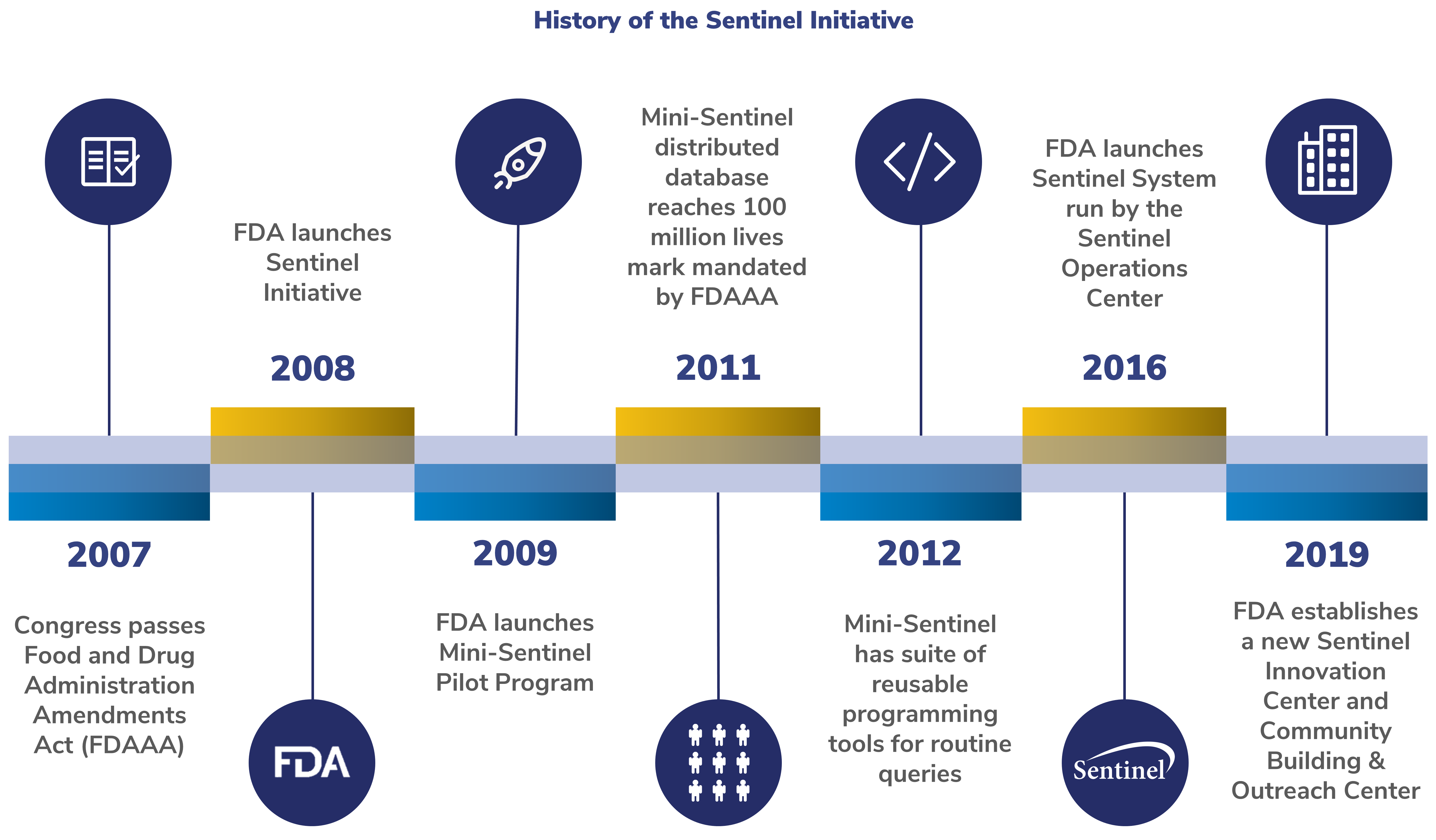
2007: Congress passes FDAAA
The FDAAA of 2007 required the FDA to create a system to assess the safety of approved medical products. The FDA was asked to work with the public, academic, and private entities. This system was to use existing electronic healthcare data from many sources.
2008: FDA launches Sentinel Initiative
In response to FDAAA, the FDA launched the Sentinel Initiative. The Sentinel Initiative aims to improve how FDA monitors medical product safety issues.
2009: FDA launches Mini-Sentinel Pilot
The Mini-Sentinel Pilot created a format for the data and a process for analyzing it. This common data model and distributed data approach enabled the FDA to:
- Track the performance of medical products
- Safeguard patient privacy
2011: Mini-Sentinel distributed dataset reaches 100 million lives mark mandated by FDAAA
2012: Mini-Sentinel launches suite of reusable programming tools for routine queries
To study safety concerns quickly, Sentinel created new programming tools to query the Sentinel Distributed Database. This allowed for Data Partners to respond in a matter of weeks.
2016: FDA launches Sentinel System
The FDA established the Active Postmarket Risk Identification and Analysis (ARIA) System. In 2016, FDA officially integrated the Sentinel System into FDA's regulatory programs.
FDA’s efforts to date have focused on developing Sentinel to serve as a tool for safety surveillance by FDA. The FDA continues to find ways to use Sentinel data in beyond safety surveillance.
2019: FDA establishes a new Sentinel Innovation Center and Community Building and Outreach Center
The Innovation Center develops innovative methods to further advance Sentinel. This includes finding ways to extract and structure information from electronic health records. Their goal is to create an analysis system with electronic health records on more than 10 million lives.
The Community Building and Outreach Center focuses on communication and collaboration. They work to deepen stakeholder involvement. This center broadens awareness, access, and use of Sentinel tools and data infrastructure.
Additionally, FDA has recruited a broad group of scientific collaborators. They contribute valuable technical support in evaluating electronic health data.
Looking for more information on the Sentinel System and Mini-Sentinel?
Read the Sentinel Program Interim Assessment and Sentinel Final Assessment Report.